Tirzepatide approved for sleep apnea treatment
MEDICATIONS
1/23/20251 min read
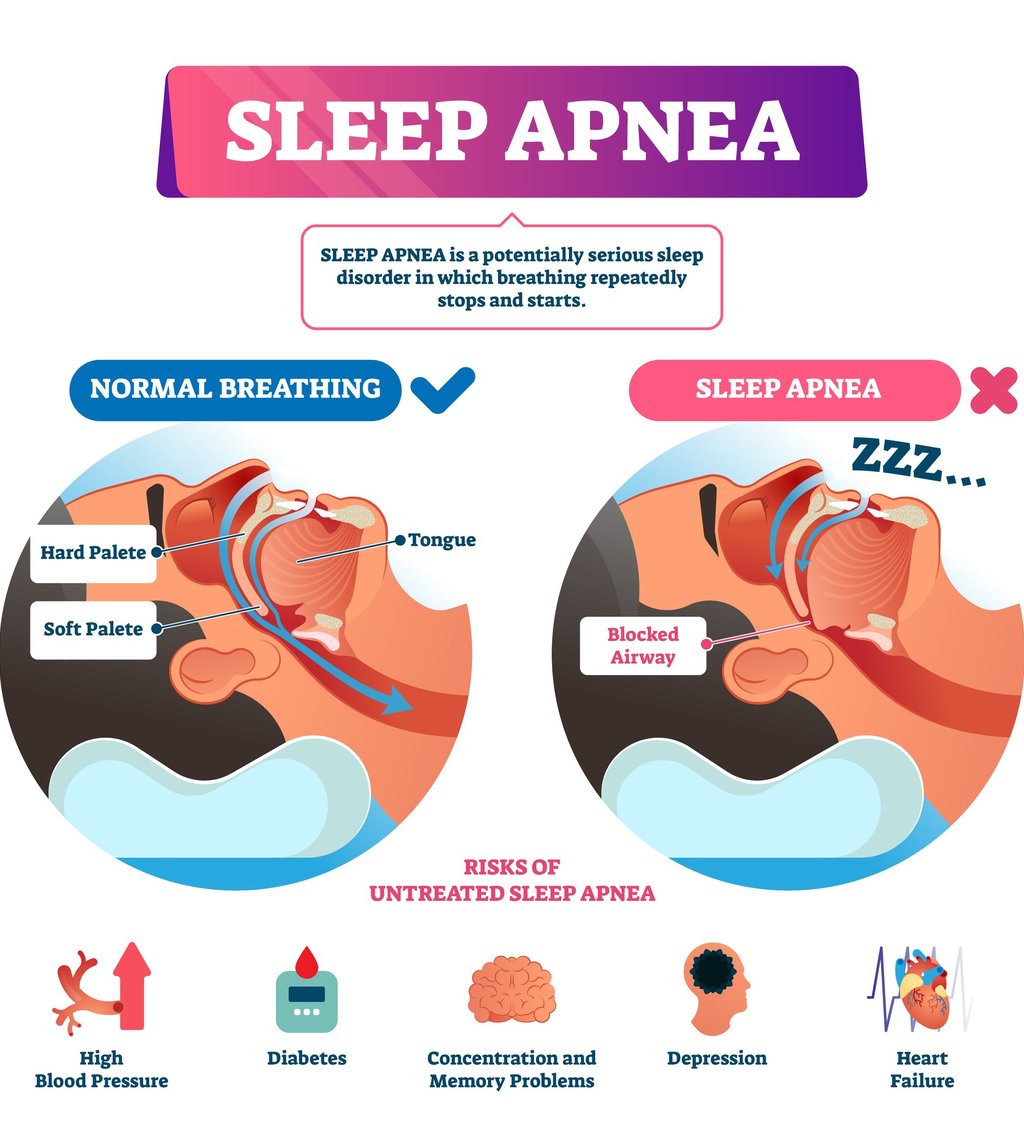
Sleep apnea can have devastating effects on your health and well-being. To make matters worse, it often leads to weight gain, which in turn can worsen sleep apnea—a vicious cycle that's difficult to escape. Until now, the primary approach to tackling this dynamic duo was through CPAP therapy to control apnea, making it easier to manage weight. But now, there’s a game-changer: we can break the cycle from both ends.
The U.S. Food and Drug Administration (FDA) has approved Eli Lilly's groundbreaking drug, Zepbound (tirzepatide), as the first medication specifically designed to treat moderate-to-severe obstructive sleep apnea (OSA) in adults with obesity.
This approval also enables Medicare Part D plans to cover Zepbound for this purpose, potentially benefiting millions of individuals struggling with the condition.
Clinical trials have shown remarkable results, with nearly half of participants experiencing significant improvements in their sleep apnea symptoms while using Zepbound.
This development represents a major advancement in the treatment of OSA, offering a new alternative to the CPAP machines that have traditionally been the mainstay of care.
2041 East St PMB 1198 Concord, CA 94520
Email: info@oparinhealth.com
WhatsApp: https://wa.me/15109071378
Instagram: https://ig.me/m/oparinhealth
Telegram: https://t.me/oparinhealth

